Atoms and Ions
Duncan Griffiths
Duncan Griffiths Copyright © april, 2003, D, GRIFFITHS, ALL RIGHTS RESERVED
This page will be a very brief overview of how atoms and Ions in water chemistry work, to be honest it is said the we are not keepers of koi, but of water, (in other words take care of the water and the koi will take care of themselves) you cannot maintain water unless a little effort is made to understand chemical reactions and their inter-relationships and it's also a nice subect to know, it can be very intimidating when hobbyists and professionals start chemical reaction jargon~~~~ ah I remember it well~~~~
So for those who are not interested? I promise, now would be a good time to hit the back button, for those who would wish they had paid more attention at school ( I include myself in this category) and would like to learn just a little more, stay with it and i will attempt to make things a little clearer, and at the end there will be a periodic table, ( a list of Atomic Elements and their symbols), this will include Atomic numbers and Atomic mass, most of them you will not need to know but I have included them because it gives the table a meaning and purpose and to do otherwise would only be doing half a Job
What is an Atom
All things are made of Elements,
examples of which are helium, oxygen, gold, iron, hydrogen, nitrogen, to name
just a few..
All elements are made up of Atoms, an Atom is the smallest part of an element
that can exist and still keep its chemical properties..
Atoms are the building
blocks of an element, all things are made of something,( an obvious statement)
just as a car is made of different parts, if we take the engine for example,
this must be made of something and it is! Namely metal elements and these
elements must be made from something, and they are, Atoms. An Atom is about
as far down the line as it goes till you analyse the structure of the Atom,
and that's where this article is heading.
Elements are
the purist form of matter that cannot be spilt by chemical reactions. Elements
are made just from atoms.
if we differ the composition of an atom we get differing types of Element.
You could say, An Atom is an Atom , just as a car is a car ,but just like
cars; atoms differ slightly, it's these different atomic structures that makes
one
element different from another. Just as the characteristics of one car to
another is determined by the arrangements of a cars components making it different.
E.G. think of it like a brick wall in a building, the brick wall represents
an element. Think of the bricks as the Elementary Atoms, typical bricks used
can be engineering bricks, common bricks, concrete bricks, breeze blocks etc,
some or all can be used on a building construction, if we decided to build
the wall out of common bricks ourwall or element takes the characteristic
of that brick, and so our element is formed, but if we make the wall out of
breeze blocks the wall or element becomes different, so according to which
is the main choice of brick or Atom this will shape the characteristics of
the wall or the element, and thus the functionality of that element.
Take the wall example yet again. Take one brick out of the wall at a time
and ask a question, is what I have left still a wall? Yes! Continue taking
the bricks out one at a time and asking the question, do I still have a wall
? If the answer is yes,continue to take bricks away from the wall. At last
we get to the point, where we have one brick left, do we still have a wall?
No! we have a brick and that's all, so we have got to the last indivisible
part of our wall , that is our ATOM!
If we relate this to our example building wall once again, we can see that
if one wall has more bricks than the next, it can be seen that even though
the two walls are made of bricks, clearly the characteristics of each wall
will be different, the same is true of the amount of atoms of any given element
that form different molecules.
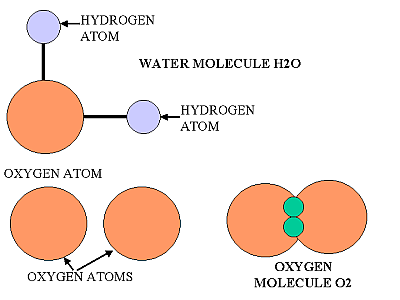
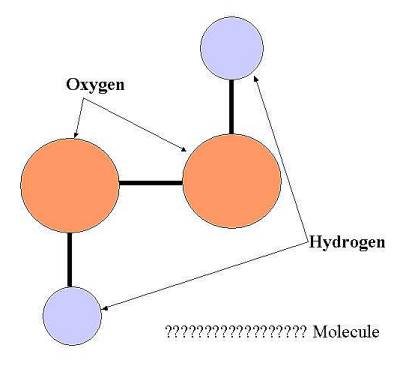
Can you work out what this is? ( answer at the bottom)
Compounds
What happens if we put several different elements together? In this event
we form a compound. A compound is several elements that CAN be split by chemical
reaction back to their original base elements
Back to our example brick wall, imagine we have our wall of common bricks
giving the characteristics of a wall made from that type of brick, but now
we want to provide insulation to that wall and make it stronger, so, along
side the original wall we build another wall out of breeze blocks close to
the original. Now we have a wall with totally different characteristics, it
has become stronger and has the new combination has given our wall insulation
properties,
This in essence is a "compound", but we can still take the newly formed wall
away leaving the original wall or element.
what
can we do with these reactions
In the koi hobby this is where we can really relate to what we have learned from the above structures.
If we combine two or more elements we form a compound and that’s what makes and shapes our water quality right through to our treatments.
For instance if we take:
1 Nitrogen atom and add 2 oxygen atoms we get nitrite, NO2
1 nitrogen atom and three oxygen atoms we form nitrate, NO3
1 nitrogen atom and three hydrogen atoms we get ammonia, NH3
1 nitrogen atom and four hydrogen atoms we get NH4, ammonium
Lets take a common pond treatment "Potassium permanganate" or KMnO4 this equals 1 part Potassium + 1 part manganese + 4 parts oxygen three elements combined together in different quantities to make a one new compoun
What is an atom made from?
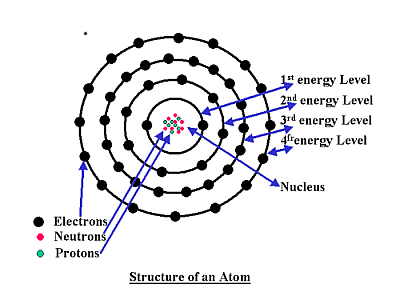
An atom consists of a central nucleus surrounded by several energy layers, the nucleus and the energy layers are commonly made up of three components namely
1, Protons
2, Neutrons
3, Electrons
The protons and the neutrons are packed into the central nucleus with the electrons occupying the outer shell energy levels. All except one element, which consists of just 1 protons and 1 electrons and NO neutrons, its name is hydrogen this is the most abundant element in the universe,. Incidentally the most common gaseous element on earth is Oxygen although oxygen only makes up 21% of the air we breath, it does however make up 47% of rocks and 89% of the oceans.
And oxygen is the second most abundant element on earth after silicone.
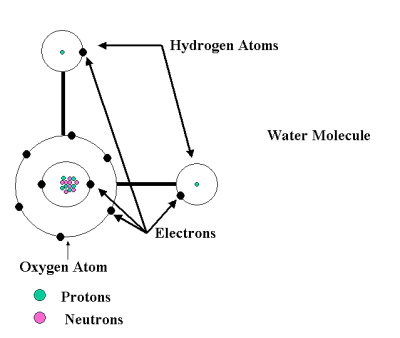
This is a diagram of what the water molecule would look like if you could see into each Atom
If we think back to our example brick wall think of these three particles protons neutrons and electrons as the components that make up the brick , (cement, sand & fine gravel)
Or, if you want to think of it another way, compare an atom to colours, there are just three primary colours, red, green and blue, these would represent protons, neutrons, and electrons, from these three primary colours a whole array of different colours are made and it’s the same with the three components that make up an atom, by arranging the numbers and the mixing of protons, neutrons and electrons we end up with a whole array of different atoms, which in turn make a whole array of elements, however pending discovery of new elements, which is unlikely there elements unlike colours are not endless, there being just over 100 of them in total.
Atomic numbers and masses
All atoms have an atomic number and an atomic mass this is determined by the arrangement and relationship of the amount of protons contained in an atom to the amount of neutrons
The atomic number represents the number of protons contained in an atom and the atomic mass is the total number of both protons and neutrons contained in that atom
So hydrogen has an atomic number of 1 and an atomic mass 1 so if we know that the atomic number is 1 and that represents the number of protons and the atomic mass is also 1 that means the Hydrogen atom must contain NO neutrons
Take the metal sodium
Atomic number = 11 that’s the number of protons it contains and the mass number for sodium is 23, (23 minus 11 = 12) so 12 is the number of neutrons sodium contains, however it is possible to get more than one type of atom for a given element and these are called Isotopes.
Oxygen is prime example of an isotope
Oxygen – 16
Oxygen – 17
Oxygen – 18
The number of protons are the same for all but the number of neutrons change
Oxygen – 16 = 8 protons this would be indicated by the atomic number 8 from 16 leaves 8 so the number of neutrons for Oxygen-16 = 8
Oxygen – 17 = 8 protons this would be indicated by the atomic number 8 from 17 leaves 9 so the number of neutrons for Oxygen-17 = 9
Oxygen – 18 = 8 protons this would be indicated by the atomic number 8 from 18 leaves 10 so the number of neutrons for Oxygen-18 = 10.
There are two isotopes of the gas Chlorine, Chlorine-35 and Chlorine-37 the atomic number for Chlorine gas is 17 so from what you now know, work out the neutron numbers for each isotope (answers at the bottom of page)
Electrons
Electrons spin around the nucleus of an atom in several energy levels giving an atom its energy, these levels follow set patterns, if we look again at the picture of the atomic structure of an atom we can see these electron levels.
The innermost energy level is called the K shell, this level can host either one or two electrons but a maximum or two electrons, then any electrons in excess of this number spill over into the next outer level called the L shell.
The second level or L shell can hold a maximum of eight electrons any electrons in excess of this will spill to the next level called the M shell.
The M shell can hold a maximum of 18 electrons, however when we get to the third M shell level, when 8 electrons occupy this level a great degree of atomic stability is achieved then electrons 9 and 10 will spill into the fourth energy level after this the third level will continue to fill to a maximum of 18 electrons
There are further energy levels containing even more numbers of electrons
The above is referred to as the electron configuration
Ions and their formation.
Another constant characteristic of an atom is , Atoms have the same amount of Protons to electrons thus Atoms have no overall charge they are neither positive + or negative -. However it is possible to find atoms with differing amount of electrons to protons and these are called Ions. Ions are formed when an atom either gains an electron or loses an electron thus altering an atoms neutral state of charge.
Its works like this, Protons are positively charged, Neutrons are neutral, and Electrons are negatively charged
So if you have the same number of protons to electrons we have in effect a stalemate with no overall charge as one cancels the other out, the neutrons, being neutral add nothing to the overall charge of the atom, but if we lose an electron or gain one this will upset the overall charge of the atom we then refer to an atom in this state as an Ion's or Cation's or more specifically anions and cations
Electrons can only be lost or gained because they are on the outside of the atoms nuclei. The nucleus remains in the same composition.
When Metals lose electrons they form a positive Ion's (or cations) now the atom has become an ion and is positively charged
Non-metals, gain electrons to become negative Ions/ or anions, but in these newly formed states we refer to these as ions and not atoms although they clearly are still atoms.
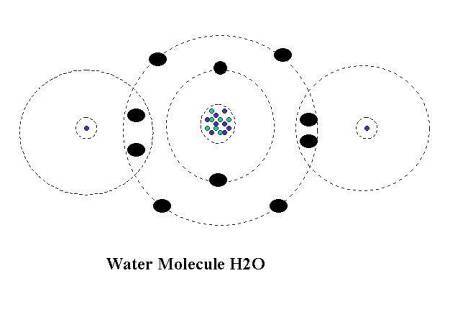
When two hydrogen atoms meet an oxygen atom they combine to share electrons
and use each others outer shell to complete there own, the hydrogen atom utilizes
one of the oxygen atoms electrons so it now has two and a complete outer shell
compliment of electrons, the other hydrogen atom does the same thing and also
now has a complete outer shell, the oxygen atom has to do the same thing but,
one Hydrogen atom will not supply it with enough electrons so it borrows the
one electron from each hydrogen atom and utilizing the one electron each hydrogen
atom has available, the oxygen atom completes its own outer level/shell and
becomes stable but they are now all bonded together and rely on each other
for atomic stability.
Some folks refer to the formula for salt as NaCl but this formula equals = (Na) sodium + (Cl) chlorine, but salt is Sodium chloride not sodium chlorine? Never the less Cl is in fact chlorine, but there is no element in the periodic table for chloride, however the formula has one subtle difference from a sodium+ chlorine NaCl, Chloride is in fact a negatively charged chlorine atom so now its know as a chloride ion"anion" , thus when we show the negativity in the formula it makes more sense the actual formula is NaCl-
Chlorine has 17 protons +18 neutrons + 17 electrons giving a neutral charge, but as chloride is an ion of the chlorine atom containing 17 protons,18 neutrons and 18 electrons, the chlorine atom has gained one electron and because the negatives outweigh the positives, the chlorine atom has change to become negatively charged chloride anion, hence the minus symbol after the Cl-
Re-cap
1, Elements are the purist form of matter that cannot be spilt by chemical reactions, containing one or several atoms
2, the smallest particle of an element that can exist is an atom
3, an atom is made up of a nucleus and outer energy levels or shells
4, protons and neutrons pack into the nucleus and electrons spin around the outer levels
5, an atom has equal number of protons to electrons, the protons are positively charged and the electrons are negatively charged thus cancelling each others charge out, the neutrons are as the name implies neutral( no charge at all)so add nothing to the overall charge of the atom.
6,when an atom loses or gains an electron the atom becomes charged.
7, an atom with a state of charge is known as an "ion", a negative charge atom is called an "anion", a positive charge atom is called a "cation".
I do hope this has helped clear things for those of you who maybe struggled a bit, if this is the case we must do this again sometime, when my brain has had the reset button pressed
Duncan
Below is the a table of common elements with there electron arrangments followed by the periodic table and of course the all important answers.
Answers,
Picture question= H2O2 hydrogen peroxide two oxygen atoms and two hydrogen atoms.
Chlorine- 35 = 18 neutrons
Chlorine - 37 = 20 neutrons
|
Element |
Atomic number |
Atomic Mass number |
Number of Protons |
Numbers of Neutrons |
Number of Electrons |
Arrangement of electrons |
|
Hydrogen H |
1 |
1 |
1 |
0 |
1 |
1 |
|
Helium He |
2 |
4 |
2 |
2 |
2 |
2 |
|
Lithium Li |
3 |
7 |
3 |
4 |
3 |
2'1 |
|
Beryllium Be |
4 |
9 |
4 |
5 |
4 |
2,2 |
|
Boron B |
5 |
11 |
5 |
6 |
5 |
2,3 |
|
Carbon C |
6 |
12 |
6 |
6 |
6 |
2,4 |
|
Nitrogen N |
7 |
14 |
7 |
7 |
7 |
2,5 |
|
Oxygen O |
8 |
16 |
8 |
8 |
8 |
2,6 |
|
Fluorine F |
9 |
19 |
9 |
10 |
9 |
2,7 |
|
Neon Ne |
10 |
20 |
10 |
10 |
10 |
2,8 |
|
Sodium Na |
11 |
23 |
11 |
12 |
11 |
2,8,1 |
|
Magnesium Mg |
12 |
24 |
12 |
12 |
12 |
2,8,2 |
|
Aluminium Al |
13 |
27 |
13 |
14 |
13 |
2,8,3 |
|
Silicon Si |
14 |
28 |
14 |
14 |
14 |
2,8,4 |
|
Phosphorus P |
15 |
31 |
15 |
16 |
15 |
2,8,5 |
|
Sulphur S |
16 |
32 |
16 |
16 |
16 |
2,8,6 |
|
Chlorine Cl |
17 |
35 |
17 |
18 |
17 |
2,8,7 |
|
Argon Ar |
18 |
40 |
18 |
22 |
18 |
2,8,8 |
|
Potassium K |
19 |
39 |
19 |
20 |
19 |
2,8,8,1 |
|
Calcium Ca |
20 |
40 |
20 |
20 |
20 |
2,8,8,2 |
|
I
|
II
|
III
|
IV
|
V
|
VI
|
VII
|
O
|
|
1
H
1 Hydrogen |
4
He Helium 2 |
|
7 Li
Lithium3 |
9 Be
Beryllium4 |
|
11 B Boron 5 |
12 C Carbon 6 |
14 N Nitrogen 7 |
16 O Oxygen 8 |
19 F Fluorine 9 |
20 Ne Neon 10 |
|||||||||
|
23 Na Sodium 11 |
24 Mg Magnesium 12 |
27 Al Aluminium 13 |
28 Si Silicon 14 |
31 P Phosphorus 15 |
32 S Sulphur 16 |
35. 5 Cl Chlorine 17 80 Br Bromine |
40 Ar Argon 18 |
||||||||||
|
39 K Potassium 19 |
40 Ca Calcium 20 |
54 Sc Scandium 21 |
48 TTitanium 22 |
51 VVanadium 23 |
52 Cr Chromium 24 |
55 Mn Manganese 25 |
56 Fe Iron 26 |
59 Co Cobalt 27 |
59 Ni Nickel 28 |
64 Cu Copper 29 |
65 Zn Zinc 30 |
70 Ga Gallium 31 |
73 Ge Germanium 32 |
75 As Arsenic 33 |
79 Se Selenium 34 |
80 Br Bromine 35 |
84 Kr Krypton 36 |
|
85. 5 Rb Rubidium 37 |
88 Sr Strontium 38 |
89 Y Yttrium 39 |
91 zr Zirconium 40 |
93 Nb Niobium 41 |
96 Mo Molybdenium 42 |
98 Tc Technetium 43 |
101 Ru Ruthenium 44 |
103 Rh Rhodium 45 |
106 Pd Palladium 46 |
108 Ag Silver 47 |
112 Cd Cadmium 48 |
115 In Indium 49 |
119 Sn Tin 50 |
122 Sb Antimony 51 |
128 Te Tellurium 52 |
127 I Iodine 53 |
131 Xe Xenon 54 |
|
133 Cs Caesium 55 |
137 Ba Barium 56 |
139 La Lanthanum 57 |
178. 5 Hf Hafnium 72 |
181 Ta Tantalum 73 |
184 W Tungsten 74 |
186 Re Rhenium 75 |
190 Os Osmium 76 |
192 Ir Iridium 77 |
195 Pt Platinum 78 |
197 Au Gold 79 |
210 Hg Mercury 80 |
204 Tl Thallium 81 |
207 Pb Lead 82 |
209 Bi Bismuth 83 |
210 Po Polonium 84 |
210 At Astatine 85 |
222 Rn Radon 86 |
|
223 Fr Francium 87 |
226 Ra Radium 88 |
227 Ac Actinium 89 |
Db Dubnium 104 |
Jl Juliotium 105 |
Rf Rutherfordium 106 |
Bh Bohrium 107 |
Hn Hahnium 108 |
Mt Meitnerium 109 |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
Symbol Name |
|
139 La Lanthaanum 57 |
140 Ce Cerium 58 |
141 Pr Praseodymium 59 |
144 Nd Neodymium 60 |
147 Pm Promethium 61 |
150 Sm Samarium 62 |
152 Eu Europium 63 |
157 Gd Gadolinium 64 |
159 Tb Terbium 65 |
162. 5 Dy Dysprosium 66 |
165 Ho Holmium 67 |
167 Er Erbium 68 |
169 Tm Thulium 69 |
173 Yb 70 |
175 Lu Lutetium 71 |
|
|
227 Ac Actinium 89 |
232 Th Thorium 90 |
231 Pa Protactinium 91 |
238 U Uranium 92 |
237 Np Neptunium 93 |
242 Pu Plutonium 94 |
243 Am Ameriicium 95 |
247 Cm Curium 96 |
247 Bk Berkelium 97 |
251 Cf Californium 98 |
254 Es Einsteinium 99 |
253 Fm Fermium 100 |
256 Md <edeleevium 101 |
254 No Nobelium 102 |
257 Lw Lawrencium 103 |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||